Standard Heat Of Formation Calcium Chloride . The standard state heat of formation for the elemental form of each atom is zero. The magnitude of δ h for a reaction depends on the physical states of the. Calcium chloride is an inorganic. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. — the elemental form of each atom is that with the lowest enthalpy in the standard state.
from www.engineeringtoolbox.com
The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. The standard state heat of formation for the elemental form of each atom is zero. — the elemental form of each atom is that with the lowest enthalpy in the standard state. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the. — standard enthalpies of formation. Calcium chloride is an inorganic. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a.
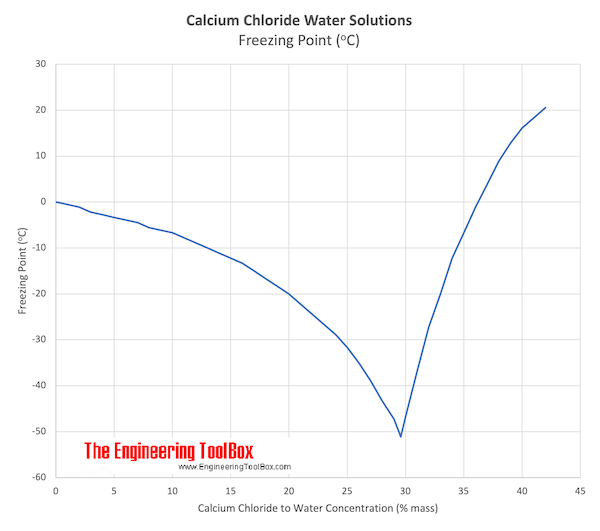
Calcium Chloride and Water
Standard Heat Of Formation Calcium Chloride — the elemental form of each atom is that with the lowest enthalpy in the standard state. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Calcium chloride is an inorganic. The magnitude of δ h for a reaction depends on the physical states of the. The standard state heat of formation for the elemental form of each atom is zero. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. — the elemental form of each atom is that with the lowest enthalpy in the standard state. — standard enthalpies of formation. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements.
From www.slideserve.com
PPT Chapters 7 and 9 PowerPoint Presentation, free download ID1947909 Standard Heat Of Formation Calcium Chloride The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — the elemental form of each atom is that with the lowest enthalpy in the standard state. The magnitude of δ h for a reaction depends on the physical states of the. 193 rows — in chemistry and thermodynamics,. Standard Heat Of Formation Calcium Chloride.
From www.vedantu.com
What is the lattice energy of Calcium Chloride? Standard Heat Of Formation Calcium Chloride The magnitude of δ h for a reaction depends on the physical states of the. Calcium chloride is an inorganic. — the elemental form of each atom is that with the lowest enthalpy in the standard state. — standard enthalpies of formation. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ. Standard Heat Of Formation Calcium Chloride.
From www.numerade.com
SOLVEDa. Use the following data to calculate the enthalpy of hydration for calcium chloride and Standard Heat Of Formation Calcium Chloride the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the. The standard state heat of formation for the elemental form of each atom is zero. The enthalpy of formation (\(δh_{f}\)) is the. Standard Heat Of Formation Calcium Chloride.
From edurev.in
Write electron dot structure for chlorine (At No. 7) and Calcium (At No.20) Show formation of Standard Heat Of Formation Calcium Chloride 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The magnitude of δ h for a reaction depends on the physical states of the. . Standard Heat Of Formation Calcium Chloride.
From www.slideserve.com
PPT BornHaber cycle for calcium chloride PowerPoint Presentation, free download ID3410019 Standard Heat Of Formation Calcium Chloride — the elemental form of each atom is that with the lowest enthalpy in the standard state. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Heat Of Formation Calcium Chloride.
From www.engineeringtoolbox.com
Calcium Chloride and Water Standard Heat Of Formation Calcium Chloride the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. — the elemental form of each atom is that with the lowest enthalpy in the standard state. The magnitude of δ h for a reaction depends on the physical states of the. 136 rows —. Standard Heat Of Formation Calcium Chloride.
From www.researchgate.net
Specific heat capacities (C sp ) measured in NaCl, KCl, CaCl 2 , and... Download Scientific Standard Heat Of Formation Calcium Chloride Calcium chloride is an inorganic. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. — the elemental form of each atom is that with the lowest enthalpy in the standard state. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data. Standard Heat Of Formation Calcium Chloride.
From slideplayer.com
Chemistry ppt download Standard Heat Of Formation Calcium Chloride — the elemental form of each atom is that with the lowest enthalpy in the standard state. Calcium chloride is an inorganic. The standard state heat of formation for the elemental form of each atom is zero. — standard enthalpies of formation. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound. Standard Heat Of Formation Calcium Chloride.
From ar.inspiredpencil.com
Calcium Chloride Lewis Structure Standard Heat Of Formation Calcium Chloride The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — the elemental form of each atom is that with the lowest enthalpy in the standard state. — standard enthalpies of formation. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h. Standard Heat Of Formation Calcium Chloride.
From dxoxcvnkx.blob.core.windows.net
Standard Heat Formation Of Magnesium Chloride at Joseph Felder blog Standard Heat Of Formation Calcium Chloride — standard enthalpies of formation. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. — the elemental form of each atom is that with the. Standard Heat Of Formation Calcium Chloride.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Standard Heat Of Formation Calcium Chloride The magnitude of δ h for a reaction depends on the physical states of the. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. —. Standard Heat Of Formation Calcium Chloride.
From kunduz.com
[ANSWERED] Using standard heats of formation, calcu... Chemistry Standard Heat Of Formation Calcium Chloride the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. The standard state heat of formation for the elemental form of each atom is zero. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance. Standard Heat Of Formation Calcium Chloride.
From learningy5li0r.z21.web.core.windows.net
How To Determine The Heat Of Formation Standard Heat Of Formation Calcium Chloride — the elemental form of each atom is that with the lowest enthalpy in the standard state. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Heat Of Formation Calcium Chloride.
From www.toppr.com
A 10.0g a sample of a mixture of calcium chloride and sodium chloride is treated with Na2CO3 to Standard Heat Of Formation Calcium Chloride Calcium chloride is an inorganic. The magnitude of δ h for a reaction depends on the physical states of the. — the elemental form of each atom is that with the lowest enthalpy in the standard state. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements. 136 rows. Standard Heat Of Formation Calcium Chloride.
From www.bartleby.com
Answered Problem 4. Calcium chloride is a salt… bartleby Standard Heat Of Formation Calcium Chloride the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. — the elemental form of each atom is that with the lowest enthalpy in the standard state. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies the formation of a compound from its elements.. Standard Heat Of Formation Calcium Chloride.
From www.numerade.com
SOLVED The reaction of calcium hydroxide (aq) with hydrochloric acid (aq) to form calcium Standard Heat Of Formation Calcium Chloride The magnitude of δ h for a reaction depends on the physical states of the. 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. — the elemental form. Standard Heat Of Formation Calcium Chloride.
From kunduz.com
[ANSWERED] 7 3 a Explain the formation of calcium chloride with the Kunduz Standard Heat Of Formation Calcium Chloride Calcium chloride is an inorganic. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. The enthalpy of formation (\(δh_{f}\)) is the enthalpy change that accompanies. Standard Heat Of Formation Calcium Chloride.
From www.showme.com
Standard heat of formation Science, Chemistry, thermochemistry ShowMe Standard Heat Of Formation Calcium Chloride 193 rows — in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 136 rows — standard enthalpy change of formation (data table) these tables include heat of formation. Standard Heat Of Formation Calcium Chloride.